Page 34 - OxyBand Research Background
P. 34
OxyBand
OxyBand™ Hydrocolloid delivery System vs. xeroform™: OxyBand TM TM
accelerated Healing and less Pain the Healing Power of Oxygen
Stanley Poulos Md, Plastic Surgery Specialists, and Marin General Hospital, Greenbrae ca. amie Franklin, Phd, OxyBand technologies inc., San Francisco, ca. Stanley Poulos Md, Plastic Surgery Spec
Pre clinical trial, the Efficacy of OxyBand™ Hydrocolloid device compared a clinical Study Evaluating
to xeroform™ on laser induced donor Sites in Healthy Volunteers OxyBand Hydrocolloid delivery System vs. xeroform™
The OxyBand™ FDA 510(K) device has been shown to speed wound The study cohort was divided into 2 groups, dressing change every 4 days
healing by delivering high concentrations of oxygen directly to the and dressing change every 5 days with follow up wound evaluation, to cOncluSiOnS OxyBand HydrOcOllOid clinical Study cli
wound bed. In addition to significantly speeding healing, human clinical confirm the optimal wear time. OxyBand™ is a multilayer wound dressing OxyBand HydrOcOllOid VS. xErOFOrM PHOtOGraPHS
trials have demonstrated significant decrease in pain and exudate. that keeps out water, dirt and germs, and supplies oxygen to the wound. • The pre clinical trial of OxyBand™, The OxyBan
Preliminary to instituting a USAISR clinical trial on donor site wounds, OxyBand™ is designed to be applied directly over clean skin or wounds and a new hydrocolloid oxygen dressing concen
with the hypothesis that treating with the OxyBand™ device will result in received its FDA 510 (k) clearance for wear time up to 5 days. A study has
for donor sites, showed that wounds
trial on donor site wounds, with the hypothesis that treating with OxyBand will result in faster
faster healing than the standard of care dressing, Xeroform™. OxyBand shown that upon attaching the dressing over a test plate, oxygen levels rise healed more quickly than with the healing
specification is a hydrocolloid interface. This preliminary trial was steadily over the device area for the first few hours and then maintain at traditional method, Xeroform™. interface. A
Hydrocolloid compared to the standard of care, Xeroform™.
conducted to evaluate the OxyBand Hydrocolloid compared to the standard elevated levels through 5 days as long as the dressing remains intact and
of care, Xeroform™. secure around the perimeter. The FDA approves providing oxygen for 5 days • Patients experienced 10 times
of continuous use. Group 1 (subjects 1-6) wounds were evaluated on Days less pain than with the traditional Methods
Methods 4 and 8, and in Group 2 (subjects 7-13) the wounds were evaluated on Day method, Xeroform™ and also
5, when the OxyBand™ dressing was removed. After Day 5, wounds were experienced enhanced patient 13 healthy human volunteers received identical burns on opposite extremities. T
13 healthy human volunteers, after obtaining appropriate informed evaluated daily without additional treatment and compared to Xeroform™. satisfaction. induced with an erbium laser set to a
consent, received identical burns on opposite extremities. Each subject The poster presents the results from comparison of the pain scores during approximately equal to a 10/10000 inch thick
the OxyBand Hydrocolloid and the other with Xeroform™ and covered with a 4x4 gauze. The
served as his or her own control. Wounds were produced with an erbium dressing changes and evaluation of wounds on Days 4 and 8 for six subjects
laser set to an ablation depth of 250 microns. This depth was selected in Group 2, Protocol 1, and Day 5 for 7 subjects in Group 2, Protocol 2. The study cohort was divided into 2 groups, 4
OxyBand is cleared by the FDA for up to 5 days. Group 1, Subjects 1-6 wounds were evaluated on
as approximately equal to a 10/10000-inch thickness donor site wound. data below was collected however the graphs show a significant difference
Wounds were treated with either OxyBand™, Hydrocolloid device, or in pain between the two groups. day 4 and day 8, and Group 2, Subjects 7-13 wounds were evaluated on day 5, when the OxyBan
Xeroform™ randomly assigned and covered with a sterile 4x4 gauze Days of Healing • Pain • Redness • Exudate • Cosmetic Appearance dressing was removed. After day 5, wounds were evaluated dail
and compared to Xeroform for, Day of Healing, Pain, Redness and Exudate. In addition, acute
dressing.
scaring, “tattooing”, and other cosmetic differences were also evaluated.
OxyBand TM 5 Day Post Op Xeroform TM 5 Day Post Op
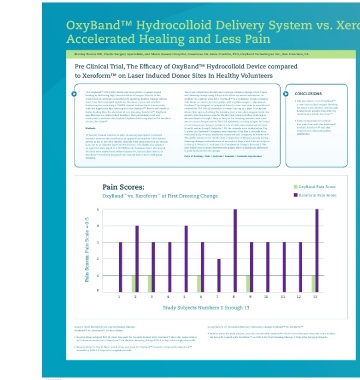
Pain Scores: OxyBand Pain Score
TM
TM
OxyBand vs. Xeroform at First Dressing Change Xeroform Pain Score
Clinical Study Results: OxyBand
5 OxyBand TM Hydrocolloid On Healing Time vs. Xeroform TM Xeroform
on Standardized 250 Micron Laser Burn Wounds
Pain Scores: Pain Scale = 0-5 3 2 Day of 100% Epithelialization 12 10 8
4
14
0 1 6 4
1 2 3 4 5 6 7 8 9 10 11 12 13 2
Study Subjects Numbers 1 through 13
0
1 2 3 4 5 6 7
Group 1 (N=6) Perceived Pain Day 8 Dressing Change; Group Two (N =7) Perceived Pain Day 5 Dressing Change OxyBand™ vs. Xeroform™
OxyBand™ Vs. Xeroform™ (24 data Points) Subject
• Results show for each subject, wounds treated with OxyBand™ felt 10 times less pain than the same subject
• Results show, subjects felt 10 times less pain for wounds treated with OxyBand™ than the same subject for wounds treated with Xeroform™ on POD 5 for First Dressing Change 5 Days after Surgica
with wounds treated with Xeroform™ on the first dressing change POD 4, 4 days after surgical wounds.
• Results show on Day 8, there was 6 times less pain for OxyBand™ wounds compared to Xeroform™
wounds on POD 8, 8 days after surgical wounds.
O2S_Poster_Second_Press_Ready.indd 1 8/12/11 12:41 PM